[Author: Laura Cowen]
US researchers have identified three plasma-soluble biomarkers that may reflect acute and chronic inflammatory states in people with fibrodysplasia ossificans progressiva (FOP).
Writing in the Journal of Bone and Mineral Research, Robert Pignolo (Mayo Clinic, Rochester, Minnesota, USA) and co-authors explain that, at present, “diagnosis of FOP is confirmed by mutation analysis, not by any single or combination of biomarkers.”
They add: “Progress toward identifying biomarkers has been impeded by the ultra-rare nature of the condition, difficulty with sample collection due to the burden and potential for harm in the collection process, as well as the logistics of timely and accurate sample collection with respect to disease activity.”
To address this, Pignolo and team screened plasma samples from 40 individuals with FOP carrying the classic ACVR1R206H mutation and 40 age- and sex-matched controls without FOP for 113 different analytes.
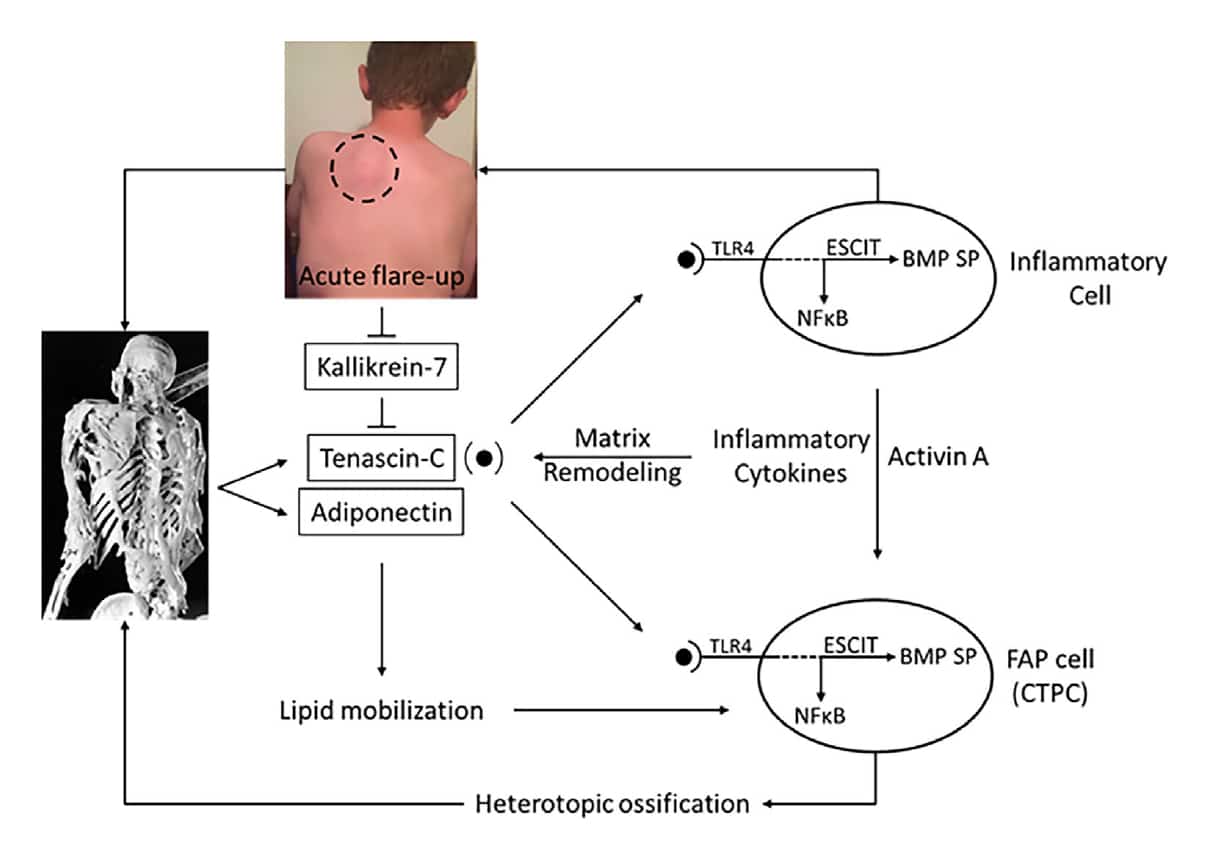
Of these, 18 were differentially expressed between people with and without FOP and two – adiponectin and tenascin-C – showed differential expression with a high degree of statistical significance.
The researchers report that adiponectin is “implicated in hypoxia, inflammation, and heterotopic ossification” and suggest that the increased levels in people with FOP versus controls may be “indicative of microenvironmental priming and susceptibility to soft tissue injury in FOP and perhaps even early (ie, preclinical) lesions.”
They add: “The finding that tenascin-C, an endogenous damage-associated molecular pattern (DAMP) that stimulates the TLR4 [toll-like receptor 4] pathway, is elevated chronically in individuals with FOP strongly suggests that chronic inflammation mediated by the TLR4 pathway is a pathophysiologic signature of FOP.”
Pignolo and colleagues also categorized the people with FOP into those with active (n=20) and inactive disease (n=20; ≥1 year since last flare up). After adjustment for age, they found that mean kallikrein-7 (KLK-7) levels were significantly lower in the people with active versus inactive FOP.
There was no significant differential expression of other biomarkers between the two groups.
The investigators note that “[t]he kallikrein-kinin system is activated in inflammation and regulates prostaglandin synthesis, suggesting that it may be involved in the early inflammatory stages of FOP lesion formation.”
Furthermore, the fact that “KLK-7 is decreased in active disease states suggests that TLR4 pro-inflammatory activity may be hyperactivated in acute flare-up states that are superimposed on a chronic inflammatory background,” they say.
Based on their findings, the authors propose a hypothetical schema (see Figure) that integrates the biomarkers they identified with the proinflammatory pathophysiology of FOP to provide a preliminary map for disease navigation.
Pignolo et al conclude that their study “identifies potential biomarkers that, with further verification, may enable the stratification of FOP stages by a minimally invasive blood draw, which poses little to no risk to individuals with FOP, and provides a real-world snapshot of both the chronic disease-related and the acute flare-up-related inflammatory nature of this complex disorder.”